1
/
of
1
Human Calmodulin-Like Protein 5 (CALML5) ELISA Kit
Human Calmodulin-Like Protein 5 (CALML5) ELISA Kit
This ELISA kit is designed to detect Human Calmodulin-Like Protein 5 (Human CALML5). The assay plate has been pre-coated with mouse anti-Human CALML5 monoclonal antibody. When the sample containing CALML5 is added to the plate, it binds to the antibodies coated on the wells. Then, a horseradish peroxidase conjugated mouse anti-Human CALML5 Antibody is added to the wells and binds to CALML5 in the sample. After washing the wells, substrate solutions are added, and the color intensity is directly proportional to the amount of Human CALML5 present. The reaction is stopped by adding an acidic stop solution, and the absorbance is measured at 450 nm.
Catalog No:
BPE224
Regular price
$754.00 USD
Regular price
$580.00 USD
Sale price
$754.00 USD
Unit price
/
per
2 weeks
Couldn't load pickup availability
Product Details
Species Reactivity
Human
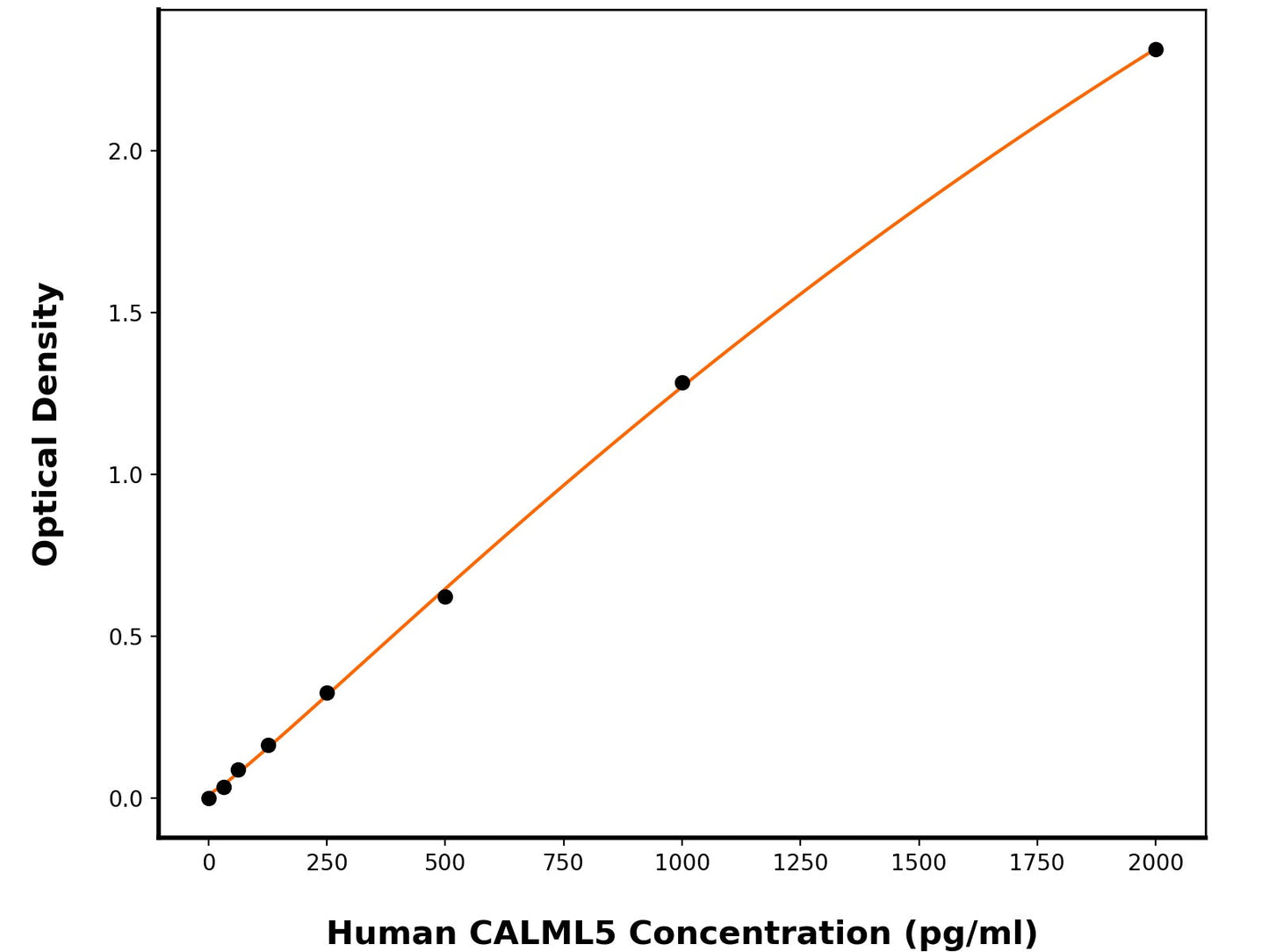
Sensitivity
6.2 pg/mL
Detection Range
31.25-2000 pg/mL
Sample Type
Serum, plasma, cell culture supernates
Incubation(s)
3.5 hour(s)
Research Areas
Cell Biology, Signal Transduction
Background
Calmodulin-like protein 5, also known as Calmodulin-like skin protein, CALML5 and CLSP, is a protein which contains fourEF-hand domains. CALML5 / CLSP is particularly abundant in the epidermis where its expression is directly related to keratinocyte differentiation.The expression is very low in lung. CALML5 / CLSP binds calcium. It may be involved in terminal differentiation of keratinocytes. Coxsackievirus and adenovirus receptor (CAR) is a member of the immunoglobulin (Ig) superfamily and a component of epithelial tight junction. CAR functions as a primary receptor for coxsackievirus B and adenovirus (Ad) infection. CALML5 / CLSP is closely related to CAR. The structure and dynamics of human calmodulin-like skin protein CALML5 / CLSP have been characterized by NMR spectroscopy. The mobility of CALML5 / CLSP has been found to be different for the N-terminal and C-terminal domains. The N-terminal domain is characterized by four stable helices, which experience large fluctuations. This is shown to be due to mutations in the hydrophobic core. The overall N-terminal domain behavior is similar both in the full-length protein and in the isolated domain.
Shipping Condition
Shipped on cold gel packs.
Storage Condition and Shelf Life
This product can be stored at 2-8C.
Analyte
Calmodulin-like protein 5
Regulatory Status
For Research Use Only